Abstract
Introduction B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T cell (CAR-T) therapy was recently approved for use in relapsed/refractory multiple myeloma (RRMM). High response rates were reported with CAR-T therapy in clinical trials and from limited real-world experiences. Currently, patients eligible for commercial CAR-T therapy are heavily pre-treated with limited therapy options. The application of commercial CAR-T therapy in RRMM is marred by several issues most importantly, access to therapy. We sought to investigate the real-world experience of outcomes of patients with RRMM on commercial CAR-T wait list since its approval in March 2021.
Methods All patients on commercial CAR-T waiting list at the University of Arkansas for Medical Sciences and Medical College of Wisconsin were included. We limited our data to the patients listed for idecabtagene vicleucel (ide-cel/ABECMA®). Clinical data was retrospectively collected with data cut off set on March 31, 2022.The cumulative incidence of CAR-T related events of interest, death, and removal from the list for other reasons over time was estimated using the Nelson-Aalen estimator. Patients who did not experience any of these events were censored at last follow-up.
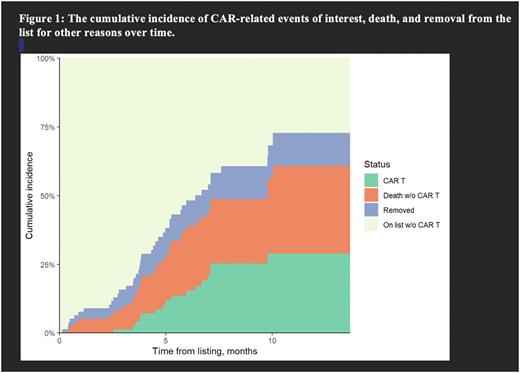
Results A total of 81 patients were added to the commercial CAR-T list between March 2021 and March 2022. The median age was 62 (range, 27-84) years with 52% being female. Ten patients (12%) were Black and 15% were out of state residents. The prior lines of therapy were 6 (2-10) and median time from diagnosis to listing for CAR-T therapy was 4.5 (range 0.8 -17.5) years. Nearly 93% patient received prior autologous stem cell transplantation (SCT) and 12% prior allogenic SCT for MM. About 90% (n=73) patients met the International Myeloma Working Group definition of progressive disease at the time of CAR-T listing. The majority (69%, n=56) of patients suffered disease relapse while waiting for apheresis. The median line of therapies after listing for patients who underwent apheresis and or received CAR T infusion was 1 (0-3). The cumulative incidence of receiving CAR-T therapy at 3-, 6- and 12-months time points were 1.34% (95% CI 0.19-9.37), 15.4% (95% CI 8.66-27.2), and 28.9% (95% CI 18.7-44.6), respectively (figure 1). The cumulative incidence of death at 12 months, while waiting for CAR-T was 31.9% (95% CI 20.5-49.5). While 12% of patients were removed, 27% remain waiting for CAR-T therapy at 12 months. The decision to remove from CAR-T listing was at the physician's discretion and included enrollment in clinical trials, procurement of CAR-T slots at an alternative center, concerns of toxicity, and diagnosis of incidental myelodysplastic disease. A total of 23 patients underwent apheresis, of which 19 were able to successfully receive CAR-T therapy. The median time to apheresis and CAR-T therapy from listing were 3.6 (range 0.9-10.3) months and 5.1 ( 2.5-9.8) months, respectively. Three patients died waiting for CAR-T manufacturing after successful apheresis and one patient is still waiting for infusion as of last follow up. Failed attempt at apheresis occurred in one patient and failed CAR-T manufacturing after successful apheresis occurred in one patient in this series.
Conclusions Among heavily pretreated RRMM patients listed for commercial BCMA CAR-T therapy, the cumulative incidence of receiving CAR-T therapy at 12 months was low at 29%. About a third of patients died waiting for CAR-T therapy in our analysis. Despite the effectiveness of CAR-T therapy in heavily pretreated MM patients in clinical trials, a limited number of patients were able to receive it in the real-world setting.
Disclosures
Schinke:Janssen: Honoraria. Dhakal:Karyopharm Therapeutics: Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Natera: Consultancy; BMS: Honoraria, Research Funding; Arcellx: Research Funding; Carsgen: Research Funding; Cartesian: Research Funding; Fate: Research Funding; Takeda: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Chhabra:Amgen: Research Funding; GlaxoSmithKline: Honoraria; Janssen: Research Funding; Sanofi: Research Funding. D'Souza:Takeda, Sanofi, TeneoBio, Prothena, Caelum Biosciences, Janssen Oncology, Regeneron, Abbvie: Research Funding; Pfizer, Janssen Oncology, Bristol-Myers Squibb/Celgene, Prothena: Consultancy, Membership on an entity's Board of Directors or advisory committees. van Rhee:Takeda: Consultancy; GlaxoSmithKline: Consultancy; Karyopharm: Consultancy; EUSA Pharma: Consultancy; Janssen Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding. Mohan:Novartis: Research Funding; BMS/Celgene: Research Funding; GSK: Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal